Building a range of targeted medicine choices for autism
Not everyone with autism wants or needs a ‘treatment’ but our patients and their families tell us that they would like the option of effective medications for distressing symptoms.
Unfortunately, clinical trials of potential drugs in autism usually fail. Participants are not carefully selected on specific characteristics and in a condition as diverse as autism, this “one size fits all” approach is unlikely to work.
We propose a more efficient approach. First, we learn from basic neuroscience research how differences in brain function occur in autism. Then, we test whether a potential medicine ‘shifts’ that brain function. If successful, we can then test the medicine in clinical trials.
Pioneering a new approach
We have begun to implement this strategy. Based on our close links with the MRC Centre for Neurodevelopmental Disorders at King's College London, which pioneers research on brain development conditions, we learned that there are differences in the brain’s chemical signalling systems in autism.
These differences were concentrated in two systems: GABA (gamma-aminobutryic acid), which dampens brain cell activity, and glutamate, which increases brain cell activity. We developed a novel study design to test for these signalling differences in individuals with autism; and found these chemical systems can be shifted.
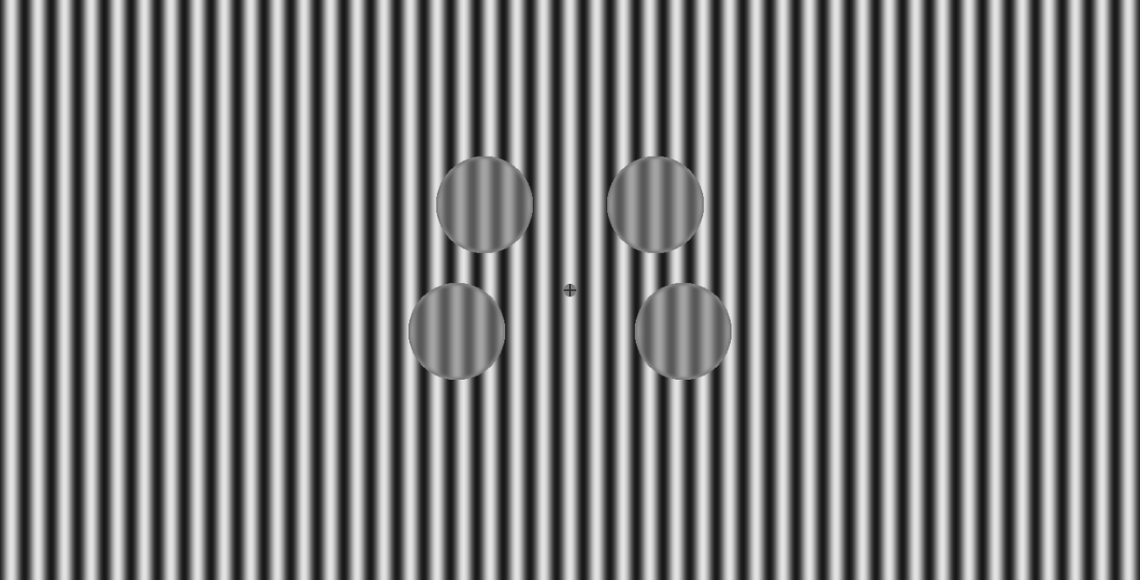
Our work highlighted GABA signalling differences as key to altered brain functioning in autism, particularly differences in processing sensory stimulation (e.g., sights - such as the visual stimulus pictured above - and sounds).
Targeting brain signalling mechanisms
Through a unique collaboration with the Simons Foundation Autism Research Initiative, and a Brain and Behaviour Research Foundation Independent Investigator award (McAlonan), we discovered that a single dose of a drug that activates the GABA system (arbaclofen) can regulate sensory processing in people with autism. We are now working together with people with lived experience of autism (A-reps) to test this drug across our 24-site Autism Clinical Trials Network in Europe.
This strategy has proved successful for other potential medicines, in work supported by the National Institute for Health and Care Research Maudsley Biomedical Research Centre and industry and now includes several medicines that target different brain signalling mechanisms.
These new medicines will target specific difficulties in autism more effectively. This will lead to more efficient clinical trials which are more likely to make medicines available in the clinic and increase choices for people with autism.
IMPACT AREAS:
Developing Resources for Research | Industry Collaboration | Personalising Treatment to Patients