Governance

The NIHR King's Clinical Research Facility (CRF) complies with Good Clinical Practice (GCP), Good Manufacturing Practice (GMP) and all relevant specific regulatory requirements and legislation.
The CRF has different approval and governance requirements depending on whether a study involves patients or healthy volunteers/non-NHS subjects.
The SLaM/IoPPN Research and Development (R&D) department or KCH R&D department can provide further information on approval requirements, for example Health Research Authority (HRA) Approval and sponsorship. You can also contact the KCL Research Ethics Office.
Combined review
Combined review is the way research teams seek approval for new Clinical Trials of Investigational Medicinal Products (CTIMPs) and combined medicine and device trials. It offers a single application route and co-ordinated review leading to a single UK decision for CTIMPs. For more information, please see the HRA website.
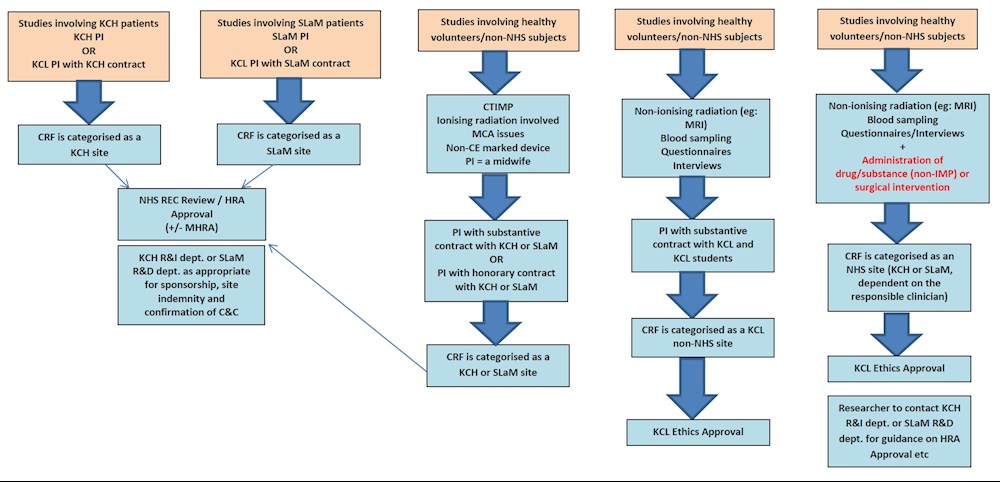
Requirements for all studies conducted in the CRF
The flowchart below summarises the requirements for all studies which are to be conducted in the CRF.
Please contact Elka Giemza, CRF Manager, if you have any questions about our governance requirements.



