Start date: Spring 2026
Application Submission Deadline: Monday 19 January 2026 (23:59 GMT)
The NIHR Maudsley Biomedical Research Centre (BRC) is inviting applications for our Introductory Clinical Research Training Scheme.
Introductory fellowships will be funded for 24 months at 0.4 FTE and will be open to KHP employed and clinically active nurses, midwives, allied health professionals (AHPs) and physician associates who wish to develop a clinical academic career in mental health or neuroscience research but have had limited research experience to date. All doctors and clinical psychologists are not eligible to apply.
The aims of the scheme are:
- To provide clinicians an opportunity to work alongside research teams, to gain a better understanding of the research process, and gain a range of research skills.
- To provide protected time for clinical health professionals to develop an application for a NIHR Pre-doctoral Award or a similar research career development award.
- To develop a cohort of research aware clinicians who will act as research champions in their clinical areas.
- To raise the profile and improve understanding of research among nurses, midwives and AHPs more widely.
Our academic partner
The appointed candidates will each work with a supervisor and research team based at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN).
The IoPPN is the largest academic community in Europe dedicated to the study, treatment and prevention of mental health problems and neurodegenerative disease. It is the world’s leading centre for mental health research in terms of citations of our publications. In the 2021 Research Excellence Framework the IoPPN was judged to have a 100% 'outstanding' (4*) world leading research environment. The impact of its research outside academia scored 92% 'outstanding' (4*) and 8% 'very considerable' (3*).
The IoPPN offers excellent opportunities for research training in basic and clinical science across the mental health spectrum including its interface with physical health, precision psychiatry, novel therapeutics and translational informatics. Studying at the IoPPN, you will benefit from world class research and clinical facilities plus internationally recognised supervisors.
We continuously strive to be an inclusive, culturally aware and culturally competent organisation that respects the differences of our community by providing an environment that both acknowledges and celebrates diversity and embraces inclusion.
Webinar
Watch: Webinar for applicants (recorded on 8 December 2025)
Watch a webinar held on 8 December for potential applicants and their clinical line managers to find out more about the programme and how to apply.
Important: Before applying for this opportunity, please read the information provided under the headings below.
This secondment scheme will allow up to four clinical health professionals to be seconded from their clinical duties part time (0.4 FTE) for 24 months. The scheme will be flexible to take account of the individual clinician’s requirements and career development. The successful candidates will remain on their current NHS contract and be paid their current NHS salary.
To be eligible for this scheme applicants must meet all the following criteria:
- Be employed as a nurse, midwife, allied health professional (AHP: NHS England » Allied health professions) or physician associate
Please note: All doctors and clinical psychologists are not eligible to apply. - Be registered with the relevant regulatory body. Below is a list of the most common eligible professional bodies, but there may be other professional registrations that are also eligible. In their application, candidates will be able to provide the name of a regulatory body not listed below.
Academy for Healthcare Science (AHCS)
British Association for Behavioural and Cognitive Psychotherapies (BABCP)
British Association for Counselling and Psychotherapy (BACP)
General Chiropractic Council (GCC)
General Dental Council (GDC)
General Medical Council (GMC)
General Optical Council (GOC)
General Osteopathic Council (GOsC)
General Pharmaceutical Council (GPhC)
Health and Care Professions Council (HCPC)
Nursing and Midwifery Council (NMC)
Social Work England
UK Council for Psychotherapists (UKCP)
UK Public Health Register
- Be clinically active.
- Be employed by one of the King’s Health Partnership NHS Foundation Trusts:
- South London & Maudsley
- King’s College Hospital
- Guy’s & St Thomas’
- Or employed by King's College London in a clinically focused role. Eg. Radiographer.
- Have been employed by their current NHS Foundation Trust / King's College London for at least 6 months.
- Have a genuine desire to develop a clinical academic career in mental health research.
- Have the support of and approval from their clinical line manager to be seconded from their clinical role for 0.4 FTE for 24 months to take up a clinical research training opportunity. It is expected that each appointee will remain working in their clinical role for the remaining NHS contractual hours.
Additionally, candidates should check the Essential and Desirable criteria listed for each of the available projects for this scheme.
Please contact us if you are unsure about your eligibility.
Stage One
To apply for this fellowship at Stage One please complete and submit an online application by 23:59 GMT on Monday 19 January 2026. The following document must be uploaded to the application:
- Line Manager Approval. Please download the Line Manager Approval form. Your Clinical Line Manager should complete this form in liaison with the relevant HR Business Partner for your post to confirm you can be released from your clinical post at 0.4 FTE for 24 months. You should then upload the completed form to accompany your online application. Applications received without this document, or with information / signatures missing from the document, will not be sent forward for shortlisting.
Project Choices Candidates must do one or both of the following:
- Select at least one and a maximum of three project choices from those listed below under Projects and explain why they are interested in applying for their chosen project/s. Candidates must contact the supervisor/s for the project/s they are interested in to discuss the project and their suitability for the placement.
- Candidates may submit their own project proposal for this scheme. To do this, please download the project proposal form and complete in collaboration with your proposed project supervisor. The completed form must be uploaded to the relevant section of the online application form.
If they wish, candidates submitting their own project proposal can also select projects from the list below as part of their application.
- Prospective applicants must discuss their proposed application with the supervisor/s for the project/s they are interested in applying for.
Referee: As part of the application, candidates are required to provide one referee; the referee should be the supervisor for the candidate’s first choice or self-designed project. A reference will only be requested if the candidate is invited to interview.
Stage Two
- Successful candidates will be invited to attend a panel interview.
- Interviews are planned for week commencing 09 February 2026 (day to be confirmed).
Following interviews, candidates will be contacted via email and informed of the outcome of their interview. It is envisaged that the successful candidates will commence their respective placements in Spring 2026.
Projects
For information about selecting projects, please refer to The application process above.
Supervisor
Dr Tom Jewell
Department of Mental Health Nursing, Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care.
Research Group: Centre for Mental Health Nursing Research | King's College London
Email: tom.1.jewell@kcl.ac.uk
Website: Thomas Jewell - King's College London
Summary of the secondment opportunity: The secondment will be based at the David Goldberg building. The secondee will join the wider mental health nursing research group based at IoPPN and the Florence Nightingale Faculty of Nursing. The secondee will be supervised by Dr Tom Jewell, who is the King’s College London lead for a 3-year research project aimed at understanding and reducing the use of restrictive practices in young people with eating disorders. This mixed methods study will provide opportunities for the fellow to gain experience of qualitative and quantitative methods, as well as patient and public involvement.
The placement will include:
- Training in reflexive thematic analysis
- Use of NVivo software
- Training in quantitative research software (SPSS and/or Stata)
- Undertaking qualitative and quantitative analyses
- Support in developing skills in writing for publication
In addition there will be opportunities to be involved in systematic reviews – developing skills in searching for literature, screening, data extraction, synthesis. There will also be opportunities to be involved in other projects, depending on the fellow’s research interests, such as a study of parent-teen interaction using video, described here:
https://www.kcl.ac.uk/news/wearable-headcams-provide-insight-complex-teen-emotions
There may also be opportunities for secondary analysis of data from this study of calories on menus and eating disorders:
The role of the secondee within the project team: The secondee will be integrated into the team for the restrictive practices project. The fellow will attend weekly research meetings, and take on a specified role for one specific sub-study within the project. Training will be provided in reflexive thematic analysis and/or quantitative research, depending on the fellow’s research interests. The fellow will then be involved in the write-up of the project and be a named author on the paper.
The secondee will also be involved in Patient and Public Involvement activities.
Estimated timelines for the secondment
In Year 1, the main focus will be developing research skills (e.g. thematic analysis). By the end of Year 1, the secondee will be a named author on at least one paper submitted to a conference. The secondee will also receive supervision and support to begin developing their own research interests ahead of applying for a pre-doctoral award.
In Year 2, the secondee will have opportunities to contribute to other projects, and will be a named co-author on at least one paper submitted for publication at a peer-reviewed journal. The secondee will also be encouraged to be involved in other research projects, based on their research interests. This could include calories on menus and eating disorders or the Eating Disorders Clinical Research Network:
https://www.kcl.ac.uk/research/eating-disorders-clinical-research-network
The secondee will also develop their own application for pre-doctoral training, supported by Tom Jewell.
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work: The secondee will have access to opportunities to be involved in the NIHR Maudsley BRC eating disorders infrastructure. Tom Jewell is a co-investigator and PPI lead for the Eating Disorders Clinical Research Network:
https://www.kcl.ac.uk/research/eating-disorders-clinical-research-network
This project also has close links with the EDGI study:
https://www.kcl.ac.uk/research/eating-disorders-genetics-initiative-edgi-1
Training and development opportunities that will be offered as part of the placement by both the project team and host department:
Opportunities include:
- Attendance at monthly mental health nursing research meetings
- In-house training in thematic analysis, quantitative analysis (desriptive statistics) and systematic reviews
- KCL training on NVivo and systematic reviews
- Attending research talks and training events at the Nursing Faculty and IoPPN.
- Attending PPI meetings and events
- The secondee will be able to attend the Qualitative Research Summer school at IoPPN:
Opportunities for patient and public involvement during the secondment: The secondee will be able to be involved in Public and Patient Involvement activities related to the restrictive practices project, particularly advisory meetings with an expert clinical advisory group. The secondee will potentially be able to make use of existing PPI networks related to Tom Jewell’s current research to inform the secondee’s own future research plans.
Expected placement outcomes
Expected outcomes at 12 months: Co-author to a poster or paper submission for a conference.
Expected outcomes at 24 months: Submission of application for external funding (e.g. NIHR predoctoral award).
Essential and desirable experience, including clinical or academic background required for the placement
Essential: An interest in eating disorders is essential. The secondee can come from any clinical background. However, the restrictive practices project would particularly suit a mental health nurse. No prior research experience is needed.
Desirable: clinical experience in eating disorders.
Supervisor
Professor Chiara Nosarti
Department of Child & Adolescent Psychiatry, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Neurodevelopment and Mental Health https://www.kcl.ac.uk/research/neurodevelopment-and-mental-health-group
Email: chiara.nosarti@kcl.ac.uk
Website: Chiara Nosarti - King's College London
Summary of the secondment opportunity: The secondment will be based at the Department of Child and Adolescent Psychiatry, IoPPN in collaboration with Professors Shaw and Sudre.
The successful secondee will receive an unparalleled training combining knowledge about childhood neurodevelopment and mental health with neuroimaging, neuro-informatics and advanced statistics. Training will be provided both (i) directly by the project, and (ii) by wider participation in the research group. Specifically, the secondee will develop skills in pipeline scripting, image processing and machine learning tools. Training will be provided through working with secondee researchers and attending courses on neuroanatomy and neuroimaging data processing and analysis. Ample career and personal development opportunities will be also offered.
The role of the secondee within the project team: The secondee will work alongside senior members of the research team in investigating the potential role of subcortico-cortical functional connectivity in emerging ADHD traits (both dimensional and meeting clinical criteria) at different developmental stages from birth to early adolescence. The secondee will be part of a larger research team but will be addressing a completely independent research question. The research topic would be ideal for a clinician interested in elucidating the etiology of ADHD, in view of developing targeted strategies for early intervention. Please see previous section for further details on knowledge and skills that will be acquired as part of this secondment.
Estimated timelines for the secondment
Year 1: Months 1-6. Training in functional MRI data processing and neuroanatomy. Months 7-12. Probing the potential role of neonatal subcortico-cortical functional connectivity in emerging ADHD traits at 18 months, 4 and 8-13 years of age in existing birth cohorts (ePRIME and Developing Human Connectome Project).
Year 2: Months 1-6. Validation of models developed in Y1 through regression models predicting ADHD traits in independent samples, including large publicly available datasets (e.g. ABCD study). Investigation of subcortico-cortical functional connectivity alterations in different subgroups (typically developing children, born preterm, with a family history of ADHD). Months 7-12. Employ the models developed in months 1-6 to develop tailored predictions of individual outcomes, within a precision psychiatry framework. Write up time, preparation of externally funded pre-doctoral career development awards to transition into more senior training research roles and progressing career in clinical research.
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The secondee will be encouraged to join the NIHR Maudsley BRC Prediction Modelling Group, which focuses on advancing prediction modelling for precision medicine and apply these methods in their project to predict ADHD symptoms using features extracted in early life from functional connectivity data. They will be also encouraged to attend regular seminar series and presentations organized by the Prediction Modelling Group.
The secondee will work in close collaboration with colleagues from the Centre for Neuroimaging Sciences, a joint venture between the Institute of Psychiatry, Psychology & Neuroscience at King's College London and South London and Maudsley NHS Foundation Trust.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department
As detailed in summary of the secondment opportunity above, the successful secondee will receive an unparalleled training combining knowledge about childhood neurodevelopment and mental health with neuroimaging, neuro-informatics and advanced statistics. Training will be provided both (i) directly by the project, and (ii) by wider participation in the research group and Department, by attending weekly lab meetings and seminars and monthly journal clubs.
In terms of project-specific skills, the secondee will develop skills in pipeline scripting, image processing and machine learning tools. Training will be provided through working with secondee researchers and attending courses on neuroanatomy and neuroimaging data processing and analysis. In addition, the secondee will receive training in research governance, including Good Clinical Practice and will be encouraged to attend research training provided within KCL Skill Forge.
The host Department will offer opportunities for interdisciplinary collaboration, and the successful secondee will be encouraged to present their work at departmental cross-disciplinary seminars and networking events. Both supervisor and Department are passionate about fostering early career researcher’s career and professional development. The successful secondee will be supportef in developing skills such as publishing papers, presenting research, and grant writing; they will be encouraged to attend conferences, join professional associations, and participate in journal clubs to build a network in the research community. When possible, we will provide some financial support for conference travel. As a supervisor, I will guide the research secondee through the process of writing papers, presenting their research and applying for funding. I will also discuss their long-term career goals, usually together with clinical academics. Both supervisor and Department believe in the importance of mental health and work-life balance and encourage open conversations about stress or challenges.
Opportunities for patient and public involvement during the secondment
We have co-produced this proposal in consultation with parents of children who have taken part in our studies, the Weston Programme for Family Centered Research (WPFCR), which involves parents to define what research is valuable to them, and to allow them to lead it with the support of academics at KCL.
At the end of the secondeeship, WPFCR members will contribute to prepare material for dissemination and to disseminate research findings, to ensure the results of the study are not just made available to academics and our participants’ families, but also to schools, child and adolescent mental health services and the wider public. Dissemination of research findings will be also facilitated by our Children’s Research Champions, an advisory board of 5 children aged 8-12 years we are working with to co-produce our research. They will be involved in ensuring child-friendly content and presentation in our social medial channels and preparing interactive science workshops in schools and in our Centre to upskill children in their understanding of neurodevelopment and mental health research. The successful secondee will be encouraged to lead some of these workshops.
Furthermore, he/she/they will attend meetings with WPFCR members twice a year, to ensure the analyses conducted as part of this project address questions that are important to the lived experience community.
Expected secondment outcomes
Expected outcomes at 12 months: Knowledge of preprocessing and analysis of functional MRI data in relation to mental health outcomes
Expected outcomes at 24 months: Named contributor to at least two published experimental papers
Essential and desirable experience, including clinical or academic background required for the secondment
Our team has extensive experience analysing paediatric neuroimaging data and training clinical and non-clinical researchers from diverse backgrounds. No previous experience with neuroimaging and complex data analysis is required, and the project would be suitable for a secondee with a clinical or an academic background.
Essential: Previous research involving analysis of quantitative data.
Desirable: Previous analysis of MRI data, research in biological psychiatry, research in neurodevelopmental disorders.
Supervisor
Dr Thomas Stephenson
Department of Forensic & Neurodevelopmental Sciences, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Forensic Research Group | King's College London
Email: thomas.stephenson@kcl.ac.uk
Website: Thomas Stephenson - King's College London
Summary of the secondment opportunity: The secondee will be based in the FANS department at IoPPN within the Forensic Research Group. A key component to the secondment will be gaining practical research experience through contributing to research activities for the SHAPE study, a mixed methods study of the role of the prison environment in self-harm behaviours amongst prisoners.
This contribution will include working on recruitment and data collection at the three study sites (two men’s category B prisons and one women’s high security prison) and leading on PPIE within the research through facilitating two existing Service User Study Advisory Groups for the study. The study is due to start recruiting in late October 2025 and study activities will continue until late 2027.
Alongside this, the secondee will be expected to prepare a proposal for their own original research during the secondment as part of preparing an application for a further research fellowship by the end of the secondment. The secondment will last between 18 and 24 months from early 2026.
The role of the secondee within the project team:
SHAPE
The secondee will be directly involved in the following activities within the SHAPE mixed methods study under close supervision. The bulk of activity will be within the quantitative component (a prospective cohort study) but the secondee will also be involved in the qualitative component (focus group study).
Recruitment: Approaching participants in prison and gaining informed consent.
Data collection: Completing questionnaires in interview format with participants, carrying out follow-up assessments at 6- and 12-weeks, extracting follow-up data from custodial and medical records for cohort study. In the focus group study, taking part in focus groups as a recorder.
Data analysis: Direct involvement in data analysis for both quantitative and qualitative components.
PPIE: Taking part in PPIE that informs the management and dissemination of the SHAPE research by facilitating meetings of existing Service User Study Advisory Group and liaising with members and host organization.
Dissemination: Leading on write-up of SHAPE secondary data analysis and involvement in write-up of main SHAPE study findings for peer-reviewed publication. Involvement in drafting of lay research summaries.
Study management: Involvement in liaising with Sponsor and other bodies for any study amendments.
Forensic Research Group: The secondee will have the opportunity to gain experience in the various activities of the Forensic Research Group within the FANS Department, including attending group and departmental meetings and spend up to 10 working days involved in research activity for other active studies in the department.
Estimated timelines for the secondment
Year 1 (starting early 2026 – prior to 1 April):
- Training (GCP)
- SHAPE recruitment & data collection (finishing early 2027)
- PPIE activities
Year 2:
- Training (in data analysis as needed via existing Forensic Mental Health MSc and NIHR Maudsley BRC resources)
- SHAPE data analysis (first half of 2027)
- PPIE activities (ending mid-Y2)
- Writing application for externally funded pre-doctoral career development award (second half of 2027)
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The secondee will join supervisor, Dr Thomas Stephenson, in taking an active part in the Prediction Modelling Group (attending monthly events, co-presenting where appropriate).
The SHAPE study findings will inform development of a clinical prediction model which the secondee can be involved in according to interest and direction of own original research proposal.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondee will be offered the following training & development opportunities:
- NIHR GCP (required)
- Training in data analysis in Stata and R by Dr Tom Stephenson and Dr Roxanna Short (lecturer, FANS)
- Research Methods module within Forensic Mental Health MSc offered by FANS department (as auditor, optional as required)
- Other data analysis training as required, such as Prediction Modelling module within Applied Statistical Modelling and Health Informatics MSc course, run by Daniel Stahl (as auditor, optional as required)
- Membership of Prediction Modelling Group
- Acting as lead facilitator for either or both Service User Study Advisory Groups (male and female) for the SHAPE study (3 meetings per year for 18 months).
Opportunities for patient and public involvement during the secondment
The secondee would have the opportunity to contribute to and, if interested, facilitate meetings of two Service User Study Advisory Groups (male and female) for the SHAPE study, taking place three times per year for the duration of the study, for the purpose of informing the management and dissemination of the research.
As part of their contribution to the group, they would be responsible for liaising with the host organization (London Offender Personality Disorder Pathway Service at London Bridge) to arrange meetings, and working with patients around managing their involvement in the group (e.g. arranging payment per NIHR rates, informing them of the requirement to report earnings to DWP if receiving benefits).
The secondee would have the opportunity to utilize the existing PPIE structure for consultation around their own new research funding proposal.
Expected secondment outcomes
Expected outcomes at 12 months:
- 3 meetings facilitated within SHAPE PPIE
- Contribution to SHAPE recruitment & data collection finished
- Co-authorship first paper of SHAPE study (qualitative)
Expected outcomes at 24 months:
- SHAPE PPIE complete
- Co-authorship of remaining SHAPE study papers
- Co-authorship with service users of lay research summaries
- Application prepared for externally funded pre-doctoral career development award
- First authorship of original research paper within SHAPE project (optional according to secondee’s interests)
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Minimum 1-year clinical experience working in NHS mental health services
Desirable
- 6-months clinical or research experience working in a forensic or prison setting
- Masters degree in relevant health science topic or equivalent research methods training
- Valid MOJ vetting approval
Supervisor
Dr Mariana Pinto da Costa
Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience.
Email: mariana.pintodacosta@kcl.ac.uk
Website: Mariana Pinto Da Costa - King's College London
Summary of the secondment opportunity: This secondment will be hosted within the Department of Psychological Medicine at the IoPPN and embedded in a NIHR research programme led by Dr Mariana Pinto da Costa, with the support of Professor Richard Emsley and Professor Robert Stewart. This is a leading clinical research environment, offering the secondee hands-on experience in conducting mental health clinical trials integrated with electronic health record (EHR) data. In the first stage the secondee will conduct a systematic review of the literature, from a transdiagnostic perspective aiming to examine how routinely collected EHR data be used to optimise trial design and improve the interpretation of real-world outcomes in mental health research. In the second stage, the secondee will support a randomised controlled trial (RCT) with people with psychosis, contributing to trial planning, data integration and extraction of the EHR-derived outcomes for the baseline trial data.
Planned activities include:
- Conducting a literature review on the use of EHRs in mental health trials.
- Extracting and analysing EHR data to generate baseline trial outcomes.
- Participating in patient recruitment
- Supporting study governance, including ethics submissions and regulatory documentation.
The secondee will work closely with the research team to gain a comprehensive understanding of the practical, regulatory, and ethical considerations involved in clinical trials and EHR-based research. Protected time will be provided to develop key research skills and prepare a competitive research funding application.
Through hands-on experience and mentorship, the secondee will acquire essential expertise in trial design, data management, and research governance, while developing a focused understanding of how EHR-informed research can address key clinical questions in mental health, enhancing their capacity to contribute to high-quality mental health research and strengthening their career trajectory.
The role of the secondee within the project team:
The secondee will be an active and integral member of the clinical research team, contributing to the design, conduct, and analysis of a mental health trial. Their work will align with the project’s central aim: to investigate how EHR data can be used to optimise clinical trial methodologies. They will contribute to a literature review, patient recruitment, EHR data extraction and analysis, and support the preparation of ethics and governance submissions, gaining first-hand experience of regulatory and ethical requirements.
Participation in team meetings, and research seminars will provide opportunities for discussion, reflection, and engagement with the wider research community. Through close mentorship from senior clinical academics, the secondee will acquire practical skills in trial planning, data management, and research governance, alongside critical thinking and scientific communication skills.
This role provides comprehensive and hands-on exposure to different stages and methods of clinical research, anchored in a transdiagnostic mental health context, equipping the secondee with the knowledge, skills, and confidence to pursue a competitive clinical academic career and make substantive contributions to advancing mental health research.
Estimated timelines for the secondment
- Months 0–6: Induction, training, literature review, familiarisation with EHR systems
- Months 6–12: Active participation in trial procedures, data collection, and preliminary analysis
- Months 12–18: Advanced data analysis, drafting manuscripts
- Months 18–24: Preparation/submission of research funding application, dissemination of findings
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
Collaborate with the Informatics Team:
- Opportunities to engage with health informatics resources
- Access to EHR datasets
Collaborate with the Clinical Trials Unit:
- Exposure to trial governance, ethics review and regulatory processes
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
- Good Clinical Practice (GCP) training
- Attendance at seminars and research meetings
- Mentorship from senior clinical academics
- Experience with research governance and ethics submission processes
- Practical training in trial recruitment, patient consent
- Training in EHR data extraction
Opportunities for patient and public involvement during the secondment
- Engagement with patient advisory panels for trial design and outcome measures
- Participation in co-developing patient-facing information
- Opportunities to present findings to PPI groups and incorporate feedback into ongoing research
Expected secondment outcomes
Expected outcomes at 12 months: Competence in systematic review conduct, trial procedures, patient recruitment, EHR-based research; understanding of research governance
Expected outcomes at 24 months: Contribution to at least one publication, submission of a research funding application, readiness to act as a research champion
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Clinically active nurse, midwife, AHP, or healthcare scientist;
- Interest in clinical research;
- Familiarity with mental health services.
Desirable
- Basic experience in research methods, data collection, or patient engagement.
Supervisor
Dr James Rucker
Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Psychoactive Trials Group | King's College London
Email: james.rucker@kcl.ac.uk
Website: James Rucker - King's College London
Summary of the secondment opportunity:
The secondment will take place at The Centre for Mental Health Research Innovation (CMHRI), a clinical research facility that operates as a collaboration between SLaM NHS Trust and KCL. The CMHRI is a purpose-built research facility specialising in the conduct of controlled clinical trials with psychedelics and related compounds such as psilocybin, MDMA and lysergic acid diethylamide (LSD). The research is conducted by The Psychoactive Trials Group (PTG), a multidisciplinary specialist team comprised of psychiatrists, psychotherapists, psychologists, nurses and academic researchers. The PTG research psychedelic therapies for various conditions including treatment resistant depression, posttraumatic stress disorder, anorexia and generalized anxiety disorder. The trials are funded from a mixture of commercial and non-commercial funders.
The aim of these trials is to explore the safety and effectiveness of these therapies. In addition, we are investigating how these new approaches could be realised in healthcare systems, developing therapist training programs and training resources for wider implementation, if they are licensed. At present, most clinical trial protocols in this field mandate the presence of at least one trained psychotherapist during drug administration, often with pre- and post-drug sessions with the same assigned therapist. The fellow will have the opportunity to support participants enrolled on psychedelic clinical trials, implementing varying degrees/models of psychological support as per each trial protocol. In addition to seeing participants across various trials, the fellow will have hands on experience supporting the research team in recruitment and retention, with opportunities for training (GCP, administration of PROs, study coordination).
The role of the secondee within the project team:
- Provide consistent psychoeducation, preparation, dosing support and integration support to participants in clinical trials, adhering to relevant trial protocols and therapy manuals.
- Communicate effectively with trial coordinators, nursing staff and medics in the creation and maintenance of a comfortable and therapeutic space for the participants and team.
- Keep contemporaneous records about therapeutic sessions and share these with the trial coordinator.
- Coordinate team supervision sessions and case review sessions.
- Attend group supervision sessions and case review sessions.
- Contribute constructively to the development of guidelines and operating procedures within the team.
- Foster overall team cohesion by negotiating and mediating team dynamics and conflicts
- Contribute to official public and patient engagement activities as appropriate, e.g. integration groups and public facing events.
- Support study coordinators and trial managers on recruitment, retention and day-to-day running of clinical trials.
- Seek relevant training opportunities for the application to a pre-doctoral training program
Estimated timelines for the secondment
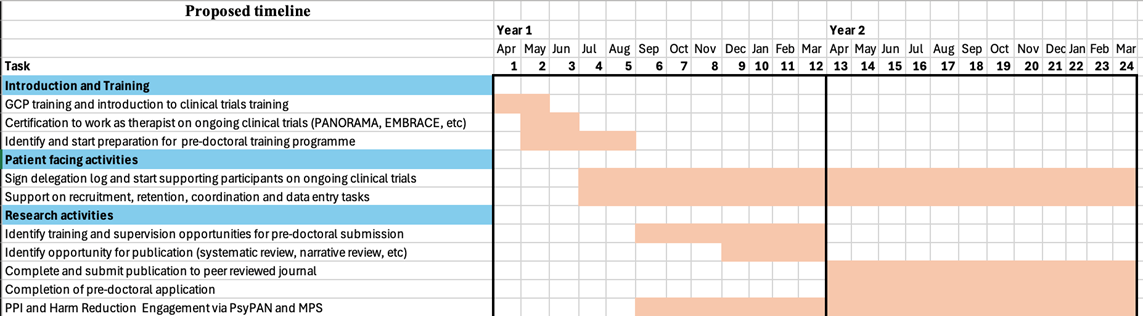
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The secondee will have the opportunity to observe and assist researchers from the wider research group (Centre for Affective Disorders) who conduct academic studies and clinical trials at the NIHR Clinical Research Facility at King’s College London. This will allow the fellow to gain experience on non-psychedelic studies that focus on treatments for bipolar disorder and other mood disorders.
The PTG have a good working relationship with the NIHR CRF and will arrange for ad-hoc shadowing days at the NIHR CRF.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
- Good Clinical Practice for CTIMPs
- Attendance of PTG weekly meetings, and Cfad monthly meetings
- Attendance of IoPPN Journal Club and Grand Rounds
- Administration of questionnaires (CROs and PROs: e.g. HAM-A, HAM-D, MADRS, GAD-7, DSST, SDS, EQ-5D-3L, STAR-C and STAR-P etc.)
- Assist study coordinators in day to day running of research projects (training in recruitment, retention, data entry)
- Assist trial managers in clinical trial management (training in ISF management, ethics applications and meetings, SLaM R&D systems (EDGE), invoicing)
- Qualitative Research Methods (via KCL)
- Quantitative Research Methods (via KCL, if desired)
- Clinical Research Protocol Development (via KCL)
- Phlebotomy, ECG and physical health assessment training (if desired)
Opportunities for patient and public involvement during the secondment
The PTG work closely with the Psychedelic Participant Advocacy Network (PsyPAN) and routinely engage with them for patient and public involvement. The fellow will be given the opportunity to coordinate ongoing PPI efforts with the group, as well as engaging with the Service User Advisory Group.
The Maudsley Psychedelic Society (MPS) is an academic interest group run by the PTG, and hosts public facing events and a monthly integration session. The MPS psychedelic integration group is open to both the public as well as previous participants of the PTG clinical trials and aims to provide a confidential and safe peer-support space to discuss the use of psychedelics. This is a harm reduction effort that does not encourage the use of psychedelics but acknowledges that if people choose to, they should do so safely. The secondee will have the opportunity to attend and/or facilitate these sessions and contribute to the organisation of lectures under the MPS.
Expected secondment outcomes
Expected outcomes at 12 months:
- Trained and certified therapist for work on ongoing clinical trials at CMHRI (LSD for GAD, Psilocybin for TRD, Psilocin for MDD)
- Actively seeing patients as part of ongoing clinical trials at PTG
- Identification of intended external pre-doctoral training program
- Initiation of research proposal / protocol development for chosen pre-doctoral training program
- Evidence of ongoing training and development relevant to pre-doctoral application
Expected outcomes at 24 months:
- Named contributor to a published paper
- Completion and submission of research proposal / application for chosen pre-doctoral training program
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Qualified accredited therapist (BACP, BABCP, HCPC), not clinical psychologist
- Experience working within an MDT
- Experience delivering flexible psychological assessment, intervention and management
- Excellent communication and interpersonal skills
- Working knowledge of Microsoft Office
- Experience with a varied and complex case load
Desirable
- Previous experience in a research environment and/or with clinical trials
- Knowledge of research landscape of drug-assisted psychotherapy research
- Experience administering diagnostic questionnaires and conducting clinical assessments
- Previous publication to a peer-reviewed academic journal
Supervisor
Dr Brendon Stubbs
Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Integrating Physical Health in Mental Disorders
Email: Brendon.stubbs@kcl.ac.uk
Website: Brendon Stubbs - King's College London
Summary of the secondment opportunity:
This secondment will be hosted within the Department of Psychological Medicine at the IoPPN and embedded in an NIHR research programme led by Dr Brendon Stubbs, with Dr Ruimin Ma as co-supervisor. The overall aim is to accelerate the development, refinement and implementation of a co-produced physical activity intervention for people with severe mental illness (SMI) and chronic pain.
Extensive patient and public involvement (PPI) and co-design work have been completed to shape the intervention. The secondee will focus on leading a series of online stakeholder events with service users, carers, clinicians, and commissioners to refine the content, format, and delivery approach. They will then support the finalisation of the intervention manual, development of a proof of concept protocol, and preparation of an ethics application (with full support).
In the second year, the secondee will contribute to delivery of the proof-of-concept study, supporting recruitment, data collection (feasibility, acceptability, and patient-reported outcomes), and dissemination.
This secondment will give the secondee hands-on experience in co-production of an intervention, stakeholder engagement, protocol development, ethics application, and feasibility testing, providing the skills and outputs to put the candidate in a strong position for an NIHR Pre-doctoral Fellowship.
The role of the secondee within the project team:
- Lead and co-facilitate online stakeholder workshops to refine the intervention.
- Support co-production of the final intervention manual.
- Draft sections of the proof-of-concept protocol and ethics submission.
- Assist in recruitment, delivery and evaluation of the proof-of-concept intervention.
- Engage service users and carers at every stage of the process.
- Contribute to dissemination through abstracts, posters, and co-authored manuscripts.
Estimated timelines for the secondment
- Months 1–3: Training (GCP, methods), review of prior co-design work, plan and deliver online stakeholder events.
- Months 4–6: Finalise intervention manual; prepare proof-of-concept protocol; draft ethics submission.
- Months 7–12: Ethics approval; begin recruitment and delivery of proof-of-concept study.
- Months 12-24: Prepare and submit an NIHR Pre Doctoral Secondment application.
- Months 13–18: Collect feasibility and acceptability data.
- Months 19–24: Analyse findings; prepare dissemination (conference abstracts, manuscript).
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
- Informatics
- Psychosis and Mood Disorders
- Trials, Genomics and Prediction
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
- Good Clinical Practice (GCP).
- Training in ethics/governance and proof of concept trial methods.
- Practical experience in stakeholder engagement and co-production.
- Opportunity to engage and assist in data analysis and receive training and support.
- Attendance at IoPPN seminars, journal clubs, BRC training sessions.
- Regular supervision from Dr Stubbs and Dr Ma.
Opportunities for patient and public involvement during the secondment
PPIE has shaped this project from the outset: service users with SMI and chronic pain have already co-designed the intervention. The secondee will continue this co-production ethos by leading stakeholder events to refine the intervention and ensure acceptability. Service users and carers will remain central to finalising materials, shaping the protocol, and interpreting findings. They will also contribute to dissemination outputs. This ensures the project remains firmly grounded in lived experience.
Expected secondment outcomes
Expected outcomes at 12 months:
- Finalised intervention manual.
- Proof-of-concept protocol complete.
- Ethics application submitted.
- Abstract submitted to national conference.
Expected outcomes at 24 months:
- Proof-of-concept intervention delivered and evaluated.
- Feasibility and acceptability data analysed.
- Named author on at least one peer-reviewed paper.
- NIHR Pre-doctoral Fellowship application submitted.
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Nurse, midwife, AHP, employed by KHP;
- Interest in clinical research;
- Commitment to patient-centred care.
Desirable
- Experience in service improvement, audit, or research activity;
- Strong communication skills;
- Confidence engaging with service users.
Supervisor
Professor Oliver Howes
Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Psychiatric Imaging | King's College London
Email: oliver.howes@slam.nhs.uk
Website: Oliver Howes - King's College London
Summary of the secondment opportunity:
60% of people with psychosis experience persistent negative symptoms, whilst treatment resistant psychotic disorders (TRP) affect about 30% of people with schizophrenia. They are associated with poorer clinical outcomes and greater health service costs.
The Maudsley Treatment Review and Assistance Team (TREAT) provides specialist intervention for people with Treatment Resistant Psychotic disorders (TRP). The service has pioneered outpatient initiation of clozapine and specialist treatments for resistant psychosis and for negative symptoms. Prior work has shown that this leads to clinical improvements, reductions in service use, and cost savings (Butler et al BJPsych 2022).
However, a number of questions remain about the real world clinical- and cost-effectiveness of outpatient clozapine initiation, and treatment approaches for negative symptoms. In particular, it is not known when the best time is to initiate clozapine for resistant psychosis, or for negative symptoms, or how to identify patients most likely to respond to intervention. This project will address these questions using real world data collected across SLaM. Depending on progress, the candidate will be able to develop this into a pragmatic clinical trial/ evaluation to assess intervention/ predictors to optimize the treatment of resistant psychosis and/or negative symptoms. This has the potential to change treatment pathways.
The project will use real-world data from patients routinely collected in TREAT service and across SLaM in terms of clinical outcomes, service use and cost efficacy. Specifically, it will examine changes in clinical measures, patient satisfaction and health care utilisation of patients in the 2-years pre- and post-intervention (either clozapine or treatment for negative symptoms). The analyses will compare the impact time to initiation of treatment on clinical, service use and cost-effectiveness outcomes, and clinical/demographic predictors of response (e.g. age, symptom profile, baseline blood data etc). Based on these findings, a pilot feasibility project will be developed in the second year to provide initial feasibility data for the most promising intervention/predictor to support a pre-doctoral fellowship or other funding proposal to test the idea further.
The role of the secondee within the project team:
The secondee will gain training in extracting data from the clinical records interactive system (CRIS), statistical analysis and the developmental of a pragmatic trial. There is the potential to contribute to the writing of manuscripts and, if interested, gaining experience within the TREAT clinical team.
They will be responsible for extracting clinical, service use and cost-effectiveness data from the TREAT research database and CRIS, analysing the data, and developing their ideas on how to evaluate the impact of clinical interventions on resistant psychosis and/or negative symptoms, including engaging with lived experience experts to gain public and patient input into the development of projects. They will also be trained in the application of large language models to interrogate reall world clinical datasets such as CRIS, and gain skills in the write-up and dissemination of scientific results. The student will also gain skills in the interpretation and development of pragmatic clinical trials to test the potential for the clinical intervention to improve patient outcomes.
Estimated timelines for the secondment
Year 1
- Months 0-3: Training in clinical data extraction from TREAT and CRIS and data analyses.
- Months 3-9: Data extraction of key clinical, service use and cost-effectiveness data
- Months 9-12: Data analyses and summarise key findings on most promising approach in terms of clinical, service use and cost outcomes
Year 2
- Months 12-15: Obtain lived experience and stakeholder input and develop pragmatic clinical trial to test most promising approach identified in year 1; develop additional TREAT/ CRIS data extraction (eg using natural language approaches) if needed, write up findings from year 1
- Months 15-18: Pilot approach and obtain preliminary evidence on feasibility trial, submit paper based on year 1 work, develop fellowship proposal for a pre-doctoral/ PhD project based on year 1 and 2 work
- Months 18-24: Analyse pilot data, finalise and submit fellowship application
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
- CRIS
- Clinical trials unit
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondee will gain training GCP and clinical research methods, including the acquisition of clinical measures, and in extracting data from the clinical records interactive system (CRIS), statistical analysis and the developmental of a pragmatic trial. When they develop the pilot feasibility component, they will also receive training in ethical applications. They will have access to training in data analyses, extraction and use of real world clinical data, as well as generic research training (statistical analyses, how to write scientific manuscripts, presentation skills, project development etc) through KCL courses.
There will also be opportunities for training in clinical trial design through the BRC/ CTU courses and clinical development through TREAT. The secondee will join the weekly group meeting to participate in peer supervision, regular supervision with the supervisors, participate in the monthly group science meeting to gain experience in presentation skills, and participate in the bimonthly group journal club to gain skills in critical appraisal. In addition, they will be able to participate in the extensive departmental activities, including bimonthly Psychosis Studies Meeting, where local, national and international speakers present the latest findings in the field, weekly student peer support meeting and opportunities to undertake modules in the departmental MSc courses (Psychiatric Research, Early Intervention, and/or Mental Health Studies).
Opportunities for patient and public involvement during the secondment
The secondee will work closely with the service user advisory group at TREAT and the Psychosis Department's Lived Experience Panel to get input into their findings, assistance with dissemination, and in the development of a potential fellowship. The analyses and recruitment will consider diversity and inclusion to ensure the representativeness of patients.
Expected secondment outcomes
Expected outcomes at 12 months:
- Final analysis of clinical, service use and cost-effectiveness data.
- Developed skills in real world data analyses.
- Developed ideas for potential fellowship project to pilot in year 2.
Expected outcomes at 24 months:
- Finalised and submitted paper(s) on year 1 analyses.
- Obtained pilot data for future project.
- Finalised and submitted proposal for external fellowship.
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- An understanding of psychosis and negative symptoms,
- An understanding of clinical record systems (eg Electronic Patient Journey)
Desirable
- An understanding of data extraction and analysis
Supervisor
Dr Ndabezinhle Mazibuko
Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Neuropharmacology Group
Email: ndabezinhle.mazibuko@kcl.ac.uk
Website: Ndabezinhle Mazibuko - King's College London
Summary of the secondment opportunity:
The secondment will take place within the Neuropharmacology Group within the Department of Neuroimaging. Trials will be undertaken at the Clinical Research Facility. The fellow will be part of a team that manages trial design, set-up, recruitment, implementation and analysis. This will include multiple clinical as well as various academic aspects of clinical trial management.
The role of the secondee within the project team:
The role will be that of a Research Assistant / Support Manager working closely with a study doctor, PhD students, other Research Assistants and the Principal Investigator.
The role is open to active nurses, midwives, allied health professionals (AHPs) and healthcare scientists who wish to develop a clinical academic career in mental health research. Clinical experience is desirable but not essential. Their role will include study design, ethics applications, study imp[lamentation and data collection, and data analysis.
Estimated timelines for the secondment
Study One (Months 0 – 12)
A Single Dose of IN Clozapine in Healthy Participants [18 participants for a minimum of 12 completers with Cerebrospinal Fluid Collection
Primary Outcome Measure
- CSF Clozapine Level (Mean and Confidence Intervals)
Secondary Outcome Measure
- EEG Measurements
Months 0-6: study design and set up
Months 6-12: study implementation and analysis
Study Two (Months 12 – 24)
A patient study, patients [15 participants for a minimum of 10 completers] who have been on oral clozapine for >2 months undergo CSF Collection.
Primary Outcome
- CSF clozapine level (Mean and Confidence Intervals)
Months 0-6 study design and set up
Months 6-12 study implementation and analysis
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The infrastructure includes The NIHR King's CRF is a purpose-built facility located at King's College Hospital which supports clinical trials in mental health, neurology, general and acute medicine.
A significant portion of the clinical trial work be conducted at this CRF.
The secondee will also have the benefit of working with the Department of Neuroimaging where the Research Group based. Recruitment and some of the latter trial work will be conducted in collaboration with SLaM Trust teams on hospital wards.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondee will have the opportunity to develop academic skills such as writing and reviewing research papers – the project will include a systemic review / meta-analysis.
There will be at least one opportunity to prepare an ethics application, attend an ethics committee, participate in the procedure for recruiting patients, in participant / patient screening at the CRF.
The secondee will be encouraged to make use of training opportunities such as GCP, conferences, journal clubs and regular academic meetings as well as shadowing the wide range of other clinical trials, learning from clinical research fellows and other academics in the department.
Opportunities for patient and public involvement during the secondment
The secondee will be involved in three interconnected studies, described below:
- The initial study involves giving a single (low) dose of healthy volunteers and then measuring their cerebrospinal fluid (CSF), fluid that surrounds the brain and spinal cord and can be sampled through a process called a lumbar puncture in the lower part of volunteer's back. We would also measure other signs of whether the clozapine was entering the brain, such as EEG measurements excessive salivation and drowsiness. So, the fellow would be working with participants recruited from the public for this study.
- The second study involves taking CSF fluid from patients. This will give us an estimate of the relationship between how much intranasal clozapine is given through the intranasal device and how much clozapine that ends up in the brain. Crucially there will be significant patient involvement
- The study design process for the patient section includes formal Patient and Public (PPI) Involvement.
Expected secondment outcomes
The fellow will be a named author on a series over up to three scientific papers.
They will have the possibility of continuing in a clinical research pathway, with the support to apply for a pre-doctoral fellowship in a related area.
Expected outcomes at 12 months:
- To help write study protocol and assist in Ethics Application and Ethics Committee attendance.
- To assist in implementation of study and data collection.
Expected outcomes at 24 months:
- To complete data collection for at least two studies.
- To assist in data analysis for at least one study.
- To have the option for continued involvement in a follow-on third study.
- To be confident in study design, protocol writing and study implementation / data collection; as well as basic relevant data analysis.
- To be confident in clinical safety and pharmacovigilance aspects in clinical trial management.
Essential and desirable experience, including clinical or academic background required for the secondment
Desirable
- Clinical experience (nursing, midwives or allied health professional); can be a healthcare scientist.
- Background within neurology, psychiatry or neuroscience would be desirable but not essential.
Supervisors
Dr Frantisek Vasa and Dr Thomas Booth
Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience.
Department of Cancer Imaging, Faculty of Life Sciences & Medicine
Research Groups: AI-Powered Portable MRI Abnormality Detection (APPMAD)
High-and Low-Field network neuroimaging laboratory (HALF-lab)
Email: frantisek.vasa@kcl.ac.uk thomas.booth@kcl.ac.uk
Website: Frantisek Vasa - King's College London Thomas Booth - King's College London
Summary of the secondment opportunity:
Recently developed ultra-low-field portable MRI systems enable brain imaging at low cost. The APPMAD project aims to develop and analytically validate an artificial intelligence (AI) tool for triaging portable MRI brain scans in adult patients into "normal" and "abnormal".
The project makes using of cutting-edge Hyperfine Swoop portable MRI systems at the King’s College London Centre for Neuroimaging Sciences and the King’s Hospital Partners- King’s College London Clinical Research Facility. Adult patients undergoing a standard clinical brain MRI scan (including T2-weighted imaging) are invited to undertake a second portable MRI scan, ideally on the same day, or no later than 30 days after the standard clinical MRI scan.
The secondee will assist with numerous aspects of the project, including patient recruitment and acquisition of brain scans on the portable MRI system. The secondee will attend project meetings regarding recruitment and scanning progress as well as research developments. The secondee will also be able to attend weekly meetings of both teams led by secondment co-supervisors, at the IoPPN and BMEIS, involving presentations, journal clubs and discussions of cutting-edge neuroimaging research.
There will be structured Training Milestones and Research Milestones to facilitate development. For example, to further the likelihood of a Pre-Doctoral Training Fellowship, we would invite opportunities for authorship and conference presentation as the secondee becomes an expert in this field.
The secondee will also have the opportunity to collaborate on closely related projects, including portable ultra-low-field neuroimaging of memory clinic patients.
The role of the secondee within the project team:
The secondee will join the APPMAD team as a radiographer on the portable ultra-low-field MRI system. They will learn about all aspects of the safe operation of the Hyperfine Swoop system, including safety, set-up of the portable MRI system, patient positioning and image acquisition. In later stages of the project the secondee will have the opportunity to learn about editing of MRI sequences on the portable MRI scanner, to create sequences with custom resolution; the performance of these sequences could be compared to “stock product” sequences with default image resolution.
Through attendance of team meetings and review of selected introductory material and research papers, the secondee will obtain a deep understanding of key steps for the pre-processing and analysis of neuroimaging data. Additionally, through these team meetings and review of recent research papers, the secondee will have the opportunity to learn about cutting edge clinical neuroimaging research.
Moreover, the secondee will have access to teaching materials from relevant KCL modules co-led by one of the secondment supervisors, on essentials of machine and deep learning approaches to neuroimaging.
Estimated timelines for the secondment
Feb 2026 – Mar 2027: Acquisition of portable KCL MRI scans at the KHP-KCL Clinical Research Facility, and KCL Centre for Neuroimaging Sciences (n = 250).
Dec 2026 – Expert at image acquisition on the Hyperfine Swoop, confident in methods for neuroimaging pre-processing and analysis (Training Milestone 1). Complete all Royal College of Radiologists AI courses (Training Milestone 2).
Mar 2027 – Begin work on NIHR Predoctoral Application.
Apr 2027 – Aug 2027: Acquisition of portable KCL MRI scans at St. Thomas’s Hospital, to serve as external validation data from a separate (but identical) portable MRI system (n = 50).
Apr 2027 – Aug 2027: Co-author on one or two published papers (Research Milestone 1). Led a PPI session (Research Milestone 2). Presentation at a conference (Research Milestone 3).
Sept 2027: Submit application for NIHR Predoctoral Award (Research Milestone 4).
Sept 2027 – Jan 2028: Fine-tuning and optimisation of custom portable MRI sequences.
Dec 2027 - Understand machine learning foundations and basic coding (Training Milestone 3).
Feb 2028: NIHR Predoctoral Award start (assuming successful).
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The work will primarily take place at the KHP-KCL Clinical Research Facility, using the KCL Hyperfine Swoop portable MRI system. Occasionally, it may not be possible to scan at the KCH CRF; in such cases scanning will instead take place at the KCL Centre for Neuroimaging Sciences (n = 250).
In later stages of the project, a small external validation dataset (n = 50) will be acquired using an identical (but separate) portable MRI system located in KCL at St. Thomas’s Hospital.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondee will receive training on all aspects of operation of the Hyperfine Swoop portable MRI system, by co-supervisors and senior radiographers working on the APPMAD project.
The secondee will be able to join weekly team meetings led by project co-leads, where they will learn about cutting edge research at the intersection of clinical neuroimaging and artificial intelligence.
The secondee will also have access to materials from a KCL module on “Machine Learning in Neuroscience”, co-led by one of the secondment supervisors, including recorded lectures and practicals in the Python programming language on essentials of machine and deep learning approaches to neuroimaging. We also expect the secondee to attend the following courses (Dr Booth is the Royal College of Radiologists AI Faculty lead and oversaw module developments):
https://my.rcr.ac.uk/event/a2GdP000000zvz3UAA/ai-in-radiology-interactive-handson-technical-course
The Hyperfine Swoop system is not a standard routine clinical scanner and everything the secondee will learn can be ‘superseded’ by leveraging their professional radiographer skillset once they have developed some experience on this research system. For example, it is expected the radiographer will become an expert and a leader in the safe operation of the Hyperfine Swoop system, including patient positioning – there are no global ‘standard operating procedures’ and radiographers are the professionals with the most experience of this skill. Other examples might be protocol development or post-processing workflows. To further the likelihood of a Pre-Doctoral Training Fellowship, authorship and conference presentation on original research pertinent to radiography, will be strongly encouraged and supported.
Maintaining GCP currency is mandatory.
Opportunities for patient and public involvement during the secondment
The APPMAD project developed a PPI focus group following meetings discussing ‘MRI and abnormality detection using AI’ with KCL’s Next Generation Medical Imaging PPI group. The group represents patients and public from throughout the UK (rural and urban), with diverse age, diverse ethnicity and diverse socio-economic status.
The secondee will have the opportunity to join future PPI / Community and Hospital Expert Involvement and Engagement (CHEIE) sessions, which have been planned and costed into the initial APPMAD funding application. It is expected that at least two sessions will take place within the 24-month duration of the secondment. We would suggest the second session is led by the Fellow.
Expected secondment outcomes
Expected outcomes at 12 months:
- Expert at image acquisition on the Hyperfine Swoop, confident in methods for neuroimaging pre-processing and analysis (Training Milestone 1).
- Identified an original research area that sits within their skillset such as procotol development, patient positioning, or post processing.
- Complete all Royal College of Radiologists AI courses (Training Milestone 2).
Expected outcomes at 24 months:
- Co-author on one or two published papers (Research Milestone 1).
- Led a PPI/ CHEIE session (Research Milestone 2).
- Presentation at a conference (Research Milestone 3).
- Understand machine learning foundations and basic coding (Training Milestone 3).
- NIHR Predoctoral Application submitted (Research Milestone 4).
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- KHP (NHS or KCL) employed
- Band 5 or above radiographer
- 2 or more years in clinical practice
- 1 or more years experience with MRI
- 1 or more years experience with neuroimaging
- Has been involved in performing imaging for a research study
Desirable
- Has taken responsibility for MRI protocol development, safety, training or similar.
- Experience of designing an audit or research study or part of a research study.
- GCP already completed
Supervisor
Professor Steve Williams
Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Centre for Neuroimaging Sciences (CNS)
Email: steve.williams@kcl.ac.uk
Website: Steven Williams - King's College London
Summary of the secondment opportunity:
This is the opportunity for an MRI radiographer with experience of conducting MRI brain examinations on patients referred from the wards and the memory clinics of the South London and Maudsley NHS Foundation Trust. The secondment would take place at the Centre for Neuroimaging Sciences and the Clinical Research Facility at the Denmark Hill campus. The secondee would champion the evaluation of motion insensitive MRI in both our clinical and research studies.
Patient motion during scanning is a serious issue in MRI, where some of the most unwell psychiatric (e.g. dementia, psychosis) and neurological (e.g. pain, movement disorder) patients struggle to remain still whilst we endeavour to scan. This often renders the images too blurred for diagnostic interpretation let alone any detailed quantitative assessment of structure.
Several approaches to minimise motion artifacts have been tried in the past (e.g. self-navigation techniques such as PROPELLER and BLADE but these techniques are limited to two-dimensional imaging). PROMO (PROspective MOtion correction) is a powerful new MR imaging strategy which digitally compensates for patient motion in all three dimensions. This introductory training scheme would allow the secondee to design and implement a series of studies to determine the clinical and research impact of PROMO on high resolution (volumetric) MRI data collection across a range of disorders.
The role of the secondee within the project team:
The candidate will lead on the design and application of novel MR imaging methods to appraise the additional value of motion insensitive imaging to delineate smaller structures such as the pituitary gland (e.g. psychosis and mood disorders), hippocampus (e.g. dementia) caudate (e.g. HD, ADHD) for a range of neurological and psychiatric indications. This will involve a comprehensive literature search of the field and recommendations for best practice in the clinical setting. They aim to champion the integration of these methods into research and hopefully inform future clinical practice. Following this initial foray into research, they hope that they might develop the requisite skill set to develop further, important research questions that might be considered for a part time higher degree in the field of neuroimaging research.
The candidate will play the central role within this project. Their main responsibilities will include optimized image collection and image analysis. This will include regular meetings with radiologists to review image quality and conspicuity with and without PROMO motion correction across datasets. The secondee will learn and apply quantitative methods to determine local and global brain tissue volumes for each sequence. This will include the application of gold standard image analysis approaches such as FreeSurfer as well as learning Matlab and Python skills. This technical focus will enable advanced neuroimaging expertise, allowing a quantitative assessment of motion artifacts in critical anatomical regions. Through introduction to this software, they will build transferable skills with future applicability in other neuroimaging research.
Beyond technical analysis, further experience in developing research ideas, ethics applications, patient recruitment, data management and patient feedback on our research findings will be gained. The candidate will identify eligible participants from existing data and will engage in participant recruitment as the study progresses. Throughout this time they will be immersed in ethics and regulatory frameworks, consolidating their knowledge of GCP and the foundations underpinning clinical research. All the while managing the time between their clinical role and the secondment project.
Combining technical skills, clinical research experience and regulatory knowledge this secondment will give the candidate a robust foundation for progressing toward independent future research in clinical neuroscience.
Estimated timelines for the secondment
The candidate will use this time to develop expertise in several aspects of MR image acquisition, statistics and analysis. Over the 24 months, they will spend 0.2 FTE of their time developing, optimising and applying image acquisition protocols (this will also incorporate any patient recruitment and additional scanning).
The other 0.2FTE will be used learning how to analyse the data from existing and emerging studies as mentioned above.
Support from the wider department will include Prof Steve Williams for applications, Prof Fernando Zelaya for physics, Dr. Owen O’Daly for statistics, Prof Gareth Barker for computing and Dr Tom Booth for radiology. Clinical collaborators include Prof Dag Aarsland (dementia), Prof Carmine Pariante (psychiatry) and Dr Daniel Van Wamelen (neurology of movement disorders).
During this time the candidate will develop the requisite skills and more specific research questions towards an application for an external pre-doctoral career development award. We fully expect the candidate to write and apply to such schemes by the end of the first 12 months and they will continue to refine their research skills whilst awaiting the outcome of such applications.
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The secondment will be based at the Centre for Neuroimaging Sciences (CNS) at KCL, the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) and the Maudsley Hospital at Denmark Hill. They will have access to a GE 3T Discovery MR750 MRI scanner at the Clinical Research Facility, as well as a 3T GE Premier research scanner and a 1.5T GE Artist for clinical scanning at the CNS.
All systems will be equipped with the necessary PROMO software to allow comprehensive evaluation in the broadest range of clinical and research settings. The secondee will also have access to the image analysis and physics teams at the CNS and to previously collected dataset of 3D volumetric scans with and without PROMO from ongoing studies.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
This secondment will offer extensive training across many domains. Advanced MRI physics and motion correction techniques with Prof Steve Williams, image analysis and quality assessment with the CNS team, statistical analysis with Dr Owen O'Daly, imaging physics with Prof Fernando Zelaya and computing and data management with Prof Gareth Barker. The candidate will gain experience optimizing protocols on our GE 3T and 1.5T scanners with PROMO capability and develop radiology interpretation skills alongside Dr Tom Booth and radiology colleagues including Dr Joerg Ederle.
There will also be an opportunity to learn Python, Matlab and image analysis tools such as FreeSurfer. GCP certification, an ethics application and protocol optimization will further reinforce fundamental facets of research expertise.
Exposure to direct experience in protocol development and participant feedback during ethics submission and patient recruitment is also planned. Additionally, a comprehensive literature review will be undertaken as well as manuscript preparation. Building conference presentation skills through departmental seminars and shadowing mock secondment interviews is also proposed.
This secondment provides significant opportunities for the candidate through multidisciplinary collaboration. They will develop project leadership and time management skills whilst receiving guided mentorship toward independent research and potential higher degree progression. Access to a breadth of ongoing and planned neuroimaging studies will further support the candidate's development.
Opportunities for patient and public involvement during the secondment
Patients will be consulted during protocol refinement to ensure the scanning procedures are designed with patient comfort and tolerability in mind. The core dementia imaging protocol is already 20 minutes long and by adding a further 5 minutes of a PROMO sequence to compare with the standard 3D volume MR imaging, the candidate is mindful of potentially exacerbating patient anxiety.
In the development of new protocols and ethics applications, the secondee will engage with relevant members of the patient community to gain their feedback regarding the content of the patient information sheet and advertisement for volunteers. The results of the study will be shared with patients such as our memory clinic participants and clinicians through a summary explaining how the improved motion correction could enhance diagnostic accuracy and reduce the need for repeated assessment and call back for repeated scans. We sincerely hope this will help patients understand the tangible benefits of the research for future clinical care.
Expected secondment outcomes
Expected outcomes at 12 months:
- Perform an audit of our most recent clinical MRI scans over the past year and derive the percentage of failed or repeated scanning required due to motion induced image artifacts. Much of this will be captured in historical radiological reports.
- Present initial findings of this audit to the department. Feedback from this presentation will help to inform future priorities regarding which scanner(s) and which patient cohorts might benefit most from the adoption of PROMO technology. An ethics submission will be developed with the appropriate managing clinicians, radiologists and participant groups.
- Perform a literature review of motion insensitive MR imaging and lead the writing of a draft manuscript for submission to the British Journal of Radiology.
- Learn how to analyse structural MRI data using the best in class software packages such as FreeSurfer.
Expected outcomes at 24 months:
- Quantitative analysis and statistical comparison of small volume measurements from conventional and PROMO sequences for a range of psychiatric and neurological patient groups.
- Present the results of this comparison during a department-wide seminar and draft a research manuscript, in collaboration with local experts for publication in a leading MR journal.
- Feedback the results from both qualitative and quantitative approaches to inform future clinical practice in the neuroimaging department.
- Early in the second year, the secondee will develop their first application for external funding with extensive support and feedback from the host department. This will be submitted, at the latest, during the first six months of the second year.
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- HCPC registered radiographer with at least 3 years MRI experience.
- Proficiency in operating MR systems, optimising complex sequences and a proven ability to troubleshoot challenging artifacts.
- Experience with advanced applications in neuroimaging
- Direct involvement in research studies and an understanding of Good Clinical Practice (GCP),
- Proven ability in MR protocol development, optimisation and standardisation
- A history of successful interdisciplinary collaboration, a proven team player and effective communicator
Desirable
- Familiarity with ethics applications, and knowledge of the principles of research governance
- Experience with phantom testing for quality assurance (QA), and a systematic approach to ensuring scan-rescan reproducibility
- Knowledge of quantitative imaging biomarkers is a significant advantage
Supervisor
Dr Sophie Bennett
Department of Psychology, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Avoidant/Restrictive Food Intake Disorder (ARFID)
Email: sophie.3.bennett@kcl.ac.uk
Website: Sophie Bennett - King's College London
Summary of the secondment opportunity:
The secondment will take place with the Avoidant/Restrictive Food Intake Disorder (ARFID) research team (KCL/SLaM – Maudsley Centre for Child and Adolescent Eating Disorders). The team has an active research programme focused on improving access to ARFID treatment for children and young people. The secondee will contribute to the two main strands of ongoing research: (1) developing and evaluating modular interventions, and (2) developing and evaluating low-intensity interventions, including those deliverable within schools.
The secondment will provide comprehensive research training, including Good Clinical Practice (GCP), and opportunities to attend additional research training within KCL. The secondee will be embedded within a research team implementing studies in clinical practice and will play an active role in reviewing literature, preparing ethics applications, assisting with recruitment, data collection, and participant follow-up and write-up. They will participate in regular academic activities such as team meetings, monthly ARFID research meetings, departmental seminars, and formal project meetings. There will also be opportunities to contribute to data analysis, manuscript preparation, and conference presentations.
The secondee will receive supervision from senior clinical academic staff, including academics and clinicians and weekly structured mentorship from Dr. Sophie Bennett to support their development. A key outcome will be planning and submitting an application for a pre-doctoral fellowship (e.g., NIHR Pre-doctoral Award) or a similar external funding opportunity.
By the end of the secondment, the secondee will have gained core research skills, contributed to innovative clinical research, and joined a network of research-active clinicians championing evidence-based approaches to ARFID treatment.
The role of the secondee within the project team:
The secondee will be supported to identify roles within the existing research programme that suit their learning needs and career aspirations. This is likely to include options to be involved in:
- Supporting ongoing systematic reviews of existing ARFID, modular and school-based interventions
- Supporting grant applications to develop/extend the low-intensity intervention work
- Informed consent for feasibility/pilot study of the newly developed modular intervention
- Supporting the development of the new modular intervention (identifying components to include, modifying the treatment based on feedback)
- Acting as a research therapist for pilot/feasibility studies, including collection of session by session measures, with supervision from a BABCP accredited CBT therapist
- Conducting qualitative interviews for the pilot/feasibility studies of the modular intervention
- Supporting quantitative and qualitative analysis with appropriate training and support write-up for the above studies.
The secondee will be supported to identify other opportunities as they arise.
Estimated timelines for the secondment
The following activities will be taking place at the relevant times of the secondment. As previously noted, the exact tasks undertaken will depend on the fellow’s training needs and aspirations.
|
Time Period |
Activity |
Details / Alignment with Project |
|
Months 1–3 (Apr–Jun 2026) |
Induction, training & integration |
Complete GCP and identify/attend relevant research training; agree learning objectives; attend ARFID research meetings, seminars and journal clubs; familiarise with existing literature, modular algorithm, and intervention development work completed to date. |
|
Months 4–9 (Jul–Dec 2026) |
Intervention development, systematic review & ethics |
Contribute to ongoing systematic reviews; support final stages of modular algorithm development; assist with ethics applications and stakeholder engagement; attend HPAG/PPI meetings every 2 months. |
|
Months 10–15 (Jan–Jun 2027) |
Pilot / feasibility study delivery & data collection |
Act as research therapist for PDSA pilot/feasibility studies; recruit participants, collect sessional outcome measures; conduct qualitative interviews with families; receive structured clinical supervision; contribute to preliminary data analysis. |
|
Months 16–21 (Jul–Dec 2027) |
Proof of concept study, analysis & fellowship preparation |
Support quantitative and qualitative data analysis; support/draft manuscripts and conference presentations; identify suitable pre-doctoral fellowship schemes and begin drafting application. |
|
Months 22–24 (Jan–Mar 2028) |
Fellowship submission & dissemination |
Finalise and submit a pre-doctoral fellowship application (e.g. NIHR); support dissemination activities from pilot and proof-of-concept work. |
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The broader team is conducting work using the Clinical Record Interactive Search (CRIS) database. The fellow will have the opportunity to learn about this work, and they may choose to use CRIS data within their application for a pre-doctoral fellowship. The team also has links with researchers in the BRC theme in Eating disorders & Obesity and the fellow will have the opportunity to make links/potentially observe researchers/research groups within this theme.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondment will provide a structured and comprehensive training programme delivered by both the ARFID research team and the IoPPN department of psychology.
The secondee will undertake core research training, including Good Clinical Practice (GCP), NIHR Research Ethics, and GDPR/safeguarding training to ensure the ethical conduct of studies with children and vulnerable populations. They will have access to KCL/relevant external research methods courses, including project management, systematic review methods (e.g. Cochrane “Conducting an Intervention Review”), and statistics and research methods, to build core research competencies.
Specialist training will support the secondee’s involvement in intervention development and pilot studies. This will include NIHR training in Patient and Public Involvement (PPI) to support co-production with children, families and professionals, informed consent with children, and implementation science and Plan Do Study Act methods training, co-production and NVIVO qualitative analysis, as well as relevant clinical training in CBT for children and adolescents to support their role as a research therapist.
The secondee will benefit from supervision and mentoring from senior clinical academics, regular ARFID team meetings, monthly research meetings, departmental seminars, and Skills Forge workshops, alongside opportunities for international collaboration with leading ARFID research groups. This integrated programme will provide the skills, experience, and leadership development necessary to support a successful pre-doctoral fellowship application and future clinical academic career.
Opportunities for patient and public involvement during the secondment
The secondee will be actively supported to develop their skills in co-production and stakeholder engagement through both formal training (e.g. NIHR PPI courses) and practical involvement.
PPI will be integrated into the intervention development phase, through collaboration with young people with ARFID, their families, and clinicians. The fellow will participate in/support delivery/facilitation of regular Health Professional Advisory Group (HPAG) and Patient and Public Involvement meetings, held approximately every two months, where stakeholder feedback informs the design, content, and delivery of modular and low-intensity interventions.
During pilot and feasibility studies, PPI input will guide recruitment strategies, ethical considerations, and acceptability assessments. Additionally, the fellow will contribute to ensuring that research outputs (e.g. publications, conference presentations, lay summaries) are accessible and meaningful to patients, families, and professionals.
Expected secondment outcomes
Expected outcomes at 12 months:
- Completion of core research training (e.g. GCP, ethics, safeguarding, GDPR, PPI, systematic review methods, relevant KCL and NIHR courses).
- Active contribution to systematic reviews and/or intervention development work, with co-authorship of a systematic review and/or protocol publication.
- Demonstrated competence in key research skills, including literature synthesis, ethics applications, participant recruitment, and data collection procedures.
- Involvement in co-production and stakeholder engagement activities (e.g. HPAG, PPI, clinician workshops).
- Attendance and at least one presentation at an internal research meeting and attendance and presentation submission for at least one local or national seminar/conference.
- Clear personal development plan agreed with supervisors, identifying the fellowship route and potential focus for the external pre-doctoral funding application.
Expected outcomes at 24 months:
- Submission of an externally funded pre-doctoral fellowship application (e.g. NIHR Pre-Doctoral Fellowship).
- First author on at least one peer-reviewed publication and potential co-author on additional outputs.
- Experience acting as a research therapist and/or qualitative interviewer in pilot or proof-of-concept studies,
- Experience collecting and analysing quantitative and/or qualitative research data.
- Contribution to grant applications and conference presentations related to ARFID modular and low-intensity interventions.
- Demonstrated ability to design and deliver research ethically with children and families, with enhanced methodological, analytic, and leadership skills.
- Clear trajectory toward doctoral research embedded in the ARFID research programme and broader clinical academic networks.
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Currently employed as a clinically active nurse, midwife, allied health professional (AHP), or healthcare scientist, working with children, young people and/or families in an NHS or equivalent clinical setting.
- Professional registration with the relevant regulatory body (e.g. NMC, HCPC).
- Demonstrable interest in developing a clinical academic career in mental health research, with enthusiasm to build research skills and experience.
- Experience working with children and young people with feeding or eating difficulties, neurodevelopmental conditions, or mental health presentations.
- Familiarity with psychological or behavioural interventions, particularly with children or families (e.g. CBT-informed approaches).
- Some basic understanding of research principles (e.g. literature searching, evidence-based practice, or participation in service evaluations/audits).
- Good communication and interpersonal skills, with the ability to work collaboratively in a multidisciplinary research environment and engage with children, families, and professionals.
- Ability to organise time effectively and balance clinical and research commitments.
Desirable
- Previous exposure to research activities (e.g. recruitment, data collection, literature reviews, ethics submissions, or contribution to audits/service evaluations).
- Evidence of dissemination activities (e.g. posters, presentations, contributions to reports or publications).
- Interest in co-production, patient and public involvement (PPI), or intervention development.
Supervisor
Professor Richard Meiser-Stedman
Department of Psychology, Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Psychology/CAMHS Clinical Academic Group (CAG)
Email: richard.meiser-stedman@kcl.ac.uk
Website: Richard Meiser-Stedman - King's College London
Summary of the secondment opportunity:
This secondment offers an opportunity to evaluate a transdiagnostic CBT- based psychoeducational group intervention for children aged 8-11, within the Boroughs of Lambeth, Lewisham, Croydon and Southwark. The groups aim to equip children with the knowledge and skills to manage anxiety as well as improve emotional regulation. The groups are currently delivered across the Boroughs specifically for children with neurodiversity, and this project aims to expand this offer to children presenting with a broader range of emotional difficulties.
The secondee will work alongside Professor Richard Meiser-Stedman and the CAMHS service lead to design and deliver a mixed-methods evaluation, examining outcomes, reach, and implementation fidelity. Quantitative data will include pre-, post- and follow-up measures. Qualitative data will be gathered from families and children participating in the groups to explore engagement, acceptability and mechanisms of change. The secondee will also apply established implementation science frameworks to identify barriers and facilitators of engagement and delivery.
Training and supervision through the NIHR Maudsley BRC will enable the secondee to develop practical skills in research design, ethics and governance, mixed methods analysis and co-production. The secondment provides protective time to lead all stages of the evaluation, producing tangible outputs to inform service improvements and building a strong foundation for progression to an NIHR Pre-Doctoral Fellowship.
Patient and public involvement will be central to the work. A small advisory group of parents and children who have already participated in the groups will co-develop study materials, advise on accessible data collection and engagement.
The role of the secondee within the project team:
The secondee will lead the day-to-day delivery of the evaluation of the trandiagnostic CBT psychoeducational group programme for children aged 8–11 across SLaM CAMHS boroughs. Working closely with Professor Richard Meiser-Stedman (IoPPN) and the CAMHS service lead, they will coordinate project activity across sites and ensure data is collected consistently and confidentially.
They will refine the evaluation framework, develop simple tools for tracking outcomes and fidelity and liaise with clinicians to embed data collection into routine practice. The secondee will also conduct initial data analysis, with guidance from the supervisory team.
A key focus of the role will involve leading patient and public involvement (PPI) activities. The secondee will establish and coordinate a small advisory group of parents and children who have attended the groups, ensuring co-production of study materials, accessibility of data collection methods, and meaningful involvement in interpreting and disseminating findings.
Throughout the secondment, the secondee will receive tailored training through the NIHR Maudsley BRC to develop practical skills in research design, ethics and governance, statistical analysis, qualitative methods, and implementation science. The role is designed to build confidence and competence in applied clinical research, preparing the secondee to progress to an NIHR Pre-Doctoral Secondeeship or equivalent clinical-academic pathway.
Estimated timelines for the secondment
Months 1-3:
- Induction to the NIHR Maudsley BRC training Programme.
- Finalisation of the evaluation protocol and governance approvals.
- Development of data collection tools.
- Set up PPI advisory group.
Months 4-6:
- Pilot data collection in 1 Borough to refine procedures and confirm feasibility
- Attend introductory course in research design, stats and qualitative methods.
Months 7-15:
- Full data collection across all participating SLam Boroughs.
- Regular fidelity monitoring.
- PPI feedback to optimise implementation
- Monthly supervision meetings and quarterly progress reviews.
Months 16-20:
- Data cleaning and quantitative and qualitative analyses supported by supervisory team and BRC training resources.
Months 21-24:
- Preparation of final report, including easy-read summary for families, service briefing for teams. Presentation at BRC trainee showcase. Develop a draft NIHR Pre-Doctoral Secondeeship proposal to extend this line of research.
Expected outputs include:
- A concise report summarising clinical outcomes, reach and implementation learning.
- A co-produced, easy read summary for families and children, developed with the PPI advisory group.
- A fidelity and implementation brief to support a sustainable model of delivery across the Boroughs.
- A poster at the NIHR Maudsley BRC trainee showcase and submission to a relevant CAMHS conference.
The project will deliver both local service improvement and enduring research capacity. Embedding evaluation and co-production within SLaM’s early intervention offer for children experiencing anxiety and emotional dysregulation.
Findings will inform the development of scalable, equitable early intervention models across CAMHS.
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The secondee will engage with the Child and Adolescent Mental Health and Implementation Science department within the NIHR Maudsley BRC. They will access BRC training sessions in applied clinical research methods, data management, patient and public involvement and will link with the BRC statistician's department. The secondee will also participate in the BRC early career researcher network and present emerging findings at the annual BRC trainee showcase, gaining exposure to a multidisciplinary research community and opportunity for collaboration.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The secondee will receive structured supervision from Professor Richard Meiser-Stedman (IoPPN), whose research focuses on anxiety and PTSD and who has extensive experience evaluating and disseminating CBT-based interventions for children and adolescents (e.g. Meiser-Stedman et al., 2023, British Journal of Clinical Psychology) as well as serving on the Research and Implementation Board of the Children and War Foundation. His expertise in translating evidence-based psychological interventions into practice provides a strong supervisory base for this secondeeship.
There will also be regular input from the CAMHS service lead on service evaluation and implementation. Training will include BRC short courses in quantitative and qualitative methods, research governance, and data analysis, plus opportunities to shadow ongoing IoPPN studies in child anxiety. The secondee will also be encouraged to develop a funding application, abstract, and poster presentation, strengthening their academic writing and dissemination skills.
This opportunity will build on the applicant’s experience with, developing, delivering and evaluating innovative CBT informed group interventions for children and young people both in clinical and school settings, and on their leadership in embedding psychologically informed practice within CAMHS. The secondeeship will provide structured research training to formalise and extend these skills through exposure to mixed-methods design, analysis and co-production. A strong focus will be on ensuring equitable access and inclusion across diverse communities, aligning with the NIHR’s commitment to improving reach and reducing inequalities in early intervention.
Opportunities for patient and public involvement during the secondment
Patient and public involvement will be embedded throughout. An explicit focus will be on ensuring equitable access and representation within the PPI advisory group, reflecting diversity of families served across SLaM CAMHS. A small advisory group of parents and children aged 8–11 who have taken part in the groups will co-design participant materials, shape accessible data-collection tools, and provide feedback on interpretation of findings. They will also help create a visual, child-friendly summary of results for dissemination to families, schools, and CAMHS teams. The secondee will coordinate this advisory group and receive BRC training in best practice for co-production and inclusive involvement.
Expected secondment outcomes
Expected outcomes at 12 months:
- Completion of protocol, governance approvals, and initial data collection in at least one borough.
- Pilot analysis of early quantitative data.
- Establishment of active PPI advisory group.
- Presentation of preliminary findings at an internal BRC or CAMHS meeting.
Expected outcomes at 24 months:
- Completion of full mixed-methods evaluation and written report.
- Co-produced summary and fidelity brief for dissemination across SLaM.
- Poster or oral presentation at the NIHR Maudsley BRC trainee showcase and/or CAMHS conference.
- Draft NIHR Pre-Doctoral Secondeeship proposal prepared for submission.
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Qualified healthcare professional (e.g. psychologist, nurse, occupational therapist, or other AHP) employed within SLaM CAMHS.
- Experience of group clinical work with children or families.
- Interest in developing clinical-academic research skills and evaluation methods.
Desirable
- Prior involvement in service evaluation, audit, or research projects.
- Experience of CBT-informed practice or psychoeducational group delivery.
- Basic understanding of data collection or analysis tools (Excel, or equivalent).
- Commitment to co-production and inclusive practice with children and families.
Supervisor
Dr Lauren Heathcote
Department of Psychology (Health Psychology), Institute of Psychiatry, Psychology & Neuroscience.
Research Group: Health psychology and behavioural medicine | Department of Psychology | King’s College London
Email: lauren.heathcote@kcl.ac.uk
Website: Lauren Heathcote - King's College London
Summary of the secondment opportunity:
This is an exciting opportunity to collaborate with the Health Psychology Section, part of the School of Mental Health & Psychological Sciences at King’s College London based at Institute of Psychiatry, Psychology, and Neuroscience (IoPPN). The IoPPN promotes cutting-edge research and translation into practice, addressing a wide range of mental health challenges.
The Health Psychology Section is home to pioneering research in health behaviours, patient outcomes, and interventions to improve mental and physical health. The successful candidate will have the opportunity to work alongside leading academics and researchers in the field, contributing to impactful studies aimed at enhancing the psychological care of patients across different health settings.
The successful candidate will work with Dr Lauren Heathcote’s team, situated in the Health Psychology Section within Guy’s Hospital. The Heathcote Lab conducts innovative research aimed at understanding mind-body mechanisms of mental and physical health, including developing and testing mind-body interventions for cancer survivors.
The successful candidate will support clinical trial activities for a Randomised Controlled Trial of ADIE (‘Aligning Dimensions of Interoceptive Experience’) Therapy, which is a new mind-body therapy to reduce anxiety in breast cancer survivors. The successful candidate will work closely with clinicians at Guy’s Hospital and the Royal Marsden Hospital, as well as within the supportive Heathcote Lab research team comprising clinical research staff and students, to support patient screening, recruitment, and will gain insight into broader trial activities.
The role of the secondee within the project team:
The successful candidate will primarily support a Randomised Controlled Trial of ADIE (‘Aligning Dimensions of Interoceptive Experience’) Therapy, which aims to reduce anxiety in breast cancer survivors. ADIE Therapy involves combining biofeedback-based training using heartbeat-sensing equipment with psychological support to re-establish a healthy mind-body connection and reduce anxiety after breast cancer.
This role offers the opportunity to actively support patient screening, recruitment, and onboarding on the ADIE Therapy trial and to gain insight and training on all other trial activities including therapy delivery, data collection, storage, and processing, data analyses, safeguarding and risk procedures, and write-up for publication. This role will also provide opportunities to engage in co-design activities with lived experience partners and charities focused on trial optimization and dissemination.
Beyond trial activities, the secondee will be embedded within a highly collaborative and active clinical research team in the Health Psychology Section: the Heathcote Lab. They will be integrated into the lab group and will be invited to join weekly meetings that provide opportunities to develop high-level knowledge and skills in clinical health research. This includes a weekly Lab Meeting which comprises expert guest speakers presenting on health psycyhology research or Journal Clubs in which members of the team read and discuss new papers in the area of health psychology, mental health, psycho-oncology, and pain science. These meetings provide an opportunitiy to build critical thinking skills which will be foundational for career development in clinical research. The fellow will, in addition, have access to the Health Psychology Section learnning and development opportunities including fortnightly Methods Monday seminars and monthly Health Psychology Seciton Seminars which showcase cutting-edge methodological and research innovations in clinical health psychology, particularly focused on supporting mental health in people living with long-term conditions.
Lastly, the secondee will received one-to-one mentorship from Dr Lauren Heathcote (Reader in Health Psychology) focused on aligning the fellow’s goals and values with appropriate further training opportunities, including a collaborative application for the NIHR Pre-doctoral Award. Dr Heathcote leads multiple long-term health psychology research programmes, including the Wellcome-funded ENTRUST Programme to build and test mind-body mental health interventions for cancer survivors; this programme provides opportunities for the Fellow to continue in the team following the introductory fellowship should this wish to.
Estimated timelines for the secondment
- Q2 2026 – Support the launch of the ADIE Therapy clinical trial, including supporting with the set-up of relevant NHS sites.
- Q3 & Q4 2026 – Support patient recruitment in the ADIE Therapy clinical trial & prepare NIHR Pre-doctoral Career Develpoment Award submission
- Q1 2027 – Submit NIHR Pre-doctoral Career Development Award (1st attempt)
- Q2 – Q3 2027 – Support patient recruitment in the ADIE therapy trial and engage in clinical health research training opportunities including weekly Lab Meetings, fortnightly Methods Monday Seminars, monthly Health Psychology Section Seminars.
- Q4 2027 to Q2 2028 – Gain insight into and support data analysis and dissemination for the ADIE Therapy trial
- Q1 2028 - Submit NIHR Pre-doctoral Career Development Award (2nd attempt, if necessary).
Areas of the NIHR Maudsley BRC infrastructure where the secondee will have the opportunity to work
The successful candidate will have the opportunity to work across numerous NIHR Maudsley BRC research themes, including neuroimaging, digital therapeutics, trials and experimental medicine.
This work will primarily involve working at Guy’s Hospital within the Health Psychology section. The successful candidate will also support patient recruitment at NHS sites, including Guy’s Hospital and the Royal Marsden Hospital.
Training and development opportunities that will be offered as part of the secondment by both the project team and host department:
The successful candidate will have the opportunity to:
- Attend clinical meetings at relevant NHS sites to support patient recruitment.
- Attend relevant KCL courses in research to advance their research skills and to support their continued professional development.
- Attend lab meetings in the Health Psychology section to advance their understanding of research and clinical trials.
- Attend team meetings for the ENTRUST programme in the Health Psychology section to advance their understanding of research and clinical trials.
- Training from the Health Psychology team to develop and implement theory-informed, mechanistically-precise behavioural interventions.
- Attend lived experience partner advisory board meetings on the ENTRUST programme to support the co-design and co-development of research alongside individuals with lived experience.
Opportunities for patient and public involvement during the secondment
The successful candidate will have the opportunity to join monthly ENTRUST Lived Experience Partner meetings, dedicated to supporting the co-design and co-development of the ADIE Therapy clinical trial and the broader ENTRUST programme activities.
The ENTRUST Lived Experience Partners are central to decision-making on the ADIE Therapy Trial and are partners in all dissemination activities for the ENTRUST Programme on building trust in the body after cancer. The partner board is overseen by Claudia Knowles, who brings years of experience in co-design in healthcare settings and provides hands-on training to all members of the Heathcote Lab team.
Expected secondment outcomes
Expected outcomes at 12 months:
- Supporting the launch of the ADIE Therapy clinical trial, setting up relevant NHS sites and supporting the recruitment of participants in the clinical trial.
- Good Clinical Practice (GCP) certification.
- Understanding of core theoretical models and methods in health psychology, and theory-informed clinical health psychology research.
- First submission of NIHR Pre-doctoral Award.
Expected outcomes at 24 months:
- Supporting the completion, analysis and dissemination of the findings from the ADIE Therapy clinical trial.
- Understanding of trial statistical analyses procedures, including the need for and implementation of appropriate blinding.
- Identified independent area of interest within clinical health psychology research and how their skills align with progression in this area.
- Second submission of NIHR Pre-doctoral Award (if unsuccessful on first attempt).
Essential and desirable experience, including clinical or academic background required for the secondment
Essential
- Strong interest in health research that is informed by theory and focused on mechanisms
- Excellent interpersonal communication and team-working skills
- Motivated and driven to improve the lives of people diagnosed with cancer
- Excellent organisational and project management skills
- Self-motivated, proactive and takes initiative to problem-solve.
Desirable
- Experience of conducting research within an NHS setting, particularly participant recruitment
- Experience working with vulnerable populations or families impacted by cancer
- Experience of NHS ethics approvals
- Experience with Patient and Public Involvement (PPI) or co-design.
The application deadline is Monday 19 January 2026 (23:59 GMT).
All applications must be submitted using the on-line application form
Please note: Applications received via email will not be sent to the review panel for short-listing.
Interviews are planned to be held via MS Teams during the week of 09 February 2026 (day to be confirmed).
Further Information:
If you have questions about these fellowships please email us: maudsley.brc@kcl.ac.uk
Follow the NIHR Maudsley BRC on X (formerly Twitter): @nihrmaudsleybrc
Read our latest research news: https://www.maudsleybrc.nihr.ac.uk/blog/





